Abstract
There are many treatments that patients with MCL go through, including combination chemotherapy, targeted therapies, and immunotherapy. However, none of these therapy treatments are cell based. So, in 2020, scientists took this into accountability and began creating a new therapy: Tercartus. This therapy treatment is the first cell-based gene therapy approved by the FDA.
What is MCL?
Mantle Cell Lymphoma, or MCL, is a cancer that impacts the lymphatic system. Your lymphatic system is “a network of organs, vessels, and tissues that work together with a colorless, watery fluid (lymph) back into your circulatory system (your bloodstream),” according to the Cleveland Clinic. Along with ensuring the proper fats and any fat soluble vitamins are correctly absorbed into the bloodstream, and ensuring the fluid levels in the body are normal, this system works to protect your body from infections by eliminating the abnormal cells in your body using a type of white blood cell called lymphocytes. There are two types of lymphocytes: B lymphocytes, or B-cells, and T lymphocytes, T-cells. The B-cells are the antibodies. They are proteins created to kill any foreign bacteria entering the body, protecting you from infections, viruses, and diseases. The T-cells are the regulators. They regulate the body’s immune system responses and kill infected cells as well as tumor cells. In those with MCL, their B-cells are malproduced as cancerous cells, commonly forming tumors in the lymph nodes and spreading rapidly to other parts of the body. Because their B-cells are no longer functional, patients with MCL are unable to fight off the cancerous cells efficiently.
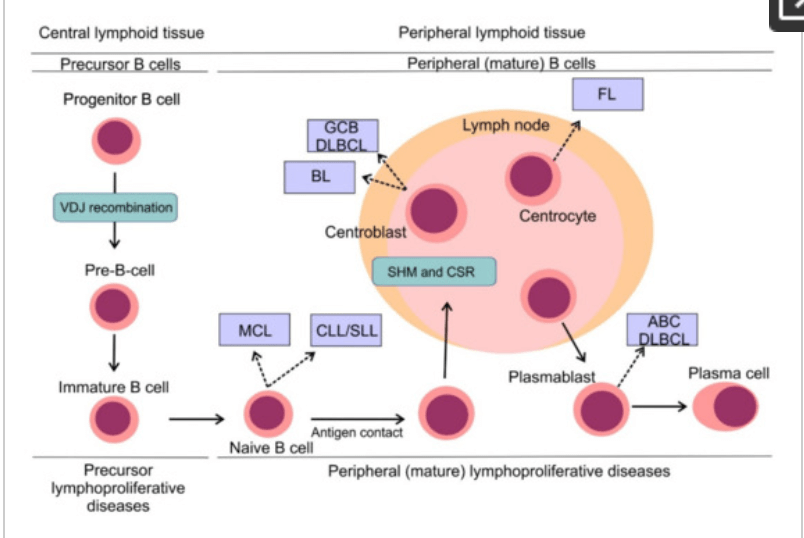
Figure from MDPI depicting the B-cell’s transformation process in those with MCL
About Tercartus
Tercartus is a treatment in which the doctor collects T-cells from the patient and genetically modifies them to kill the lymphoma cells while performing its normal functions, then infuses the white blood cell back into the body of the patient. It was first tested in a trial of 60 adults with relapsed or refractory MCL. As a result, doctors found that the complete remission rate was 62%, and held an objective response rate of 87%. In other words, all signs of cancer were eliminated in 62% of patients, and 87% of patients experienced a decrease in cancerous cells using this treatment. Using this genetically modifying treatment would mitigate the risks in some other treatments. For example, chemotherapy is a process in which radiation is used to kill the cancerous cells in the patient. However, there is little accuracy to have the radiation only kill the cancer cells specifically, resulting in the radiation also killing other functional cells that may benefit the body. Using Tercartus, only the cancer cells will be targeted, allowing the other cells in the body to remain unharmed. It adds another level of accuracy and security when eliminating cancerous cells.
Side Effects and Risks
However, like any treatment in the works, Tercartus does have its side effects. Some patients experienced serious infection, reduced blood cell count, and a weakened immune system. The treatment comes with a boxed warning for cytokine release syndrome (CRS), which is a systematic response to the creation and reproduction of Chimeric Antigen Receptor (CAR) T cells. This is dangerous as the sudden production of CAR-T cells can cause high fever and flu-like symptoms, which could be fatal and life-threatening. Nonetheless, this treatment was approved by the FDA in risk evaluation and mitigation strategies, including ETASU (elements to assure safe use). Tercartus is a very promising procedure, allowing the treatment to be approved under accelerated approval pathways and priority review.
References
https://my.clevelandclinic.org/health/body/21199-lymphatic-system
Image reference: https://www.mdpi.com/2072-6694/12/4/938
Leave a comment